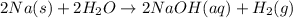"The reactivity of each sample with water" is sufficient to differentiate the sample of sodium from a sample of silver.
Explanation :
Silver does not undergo any reaction with water. Also, silver is insoluble in water under normal conditions. Thus it remains in its metallic state when it comes in contact with water.
On the other hand, sodium is highly reactive in water. It forms a colorless solution in water, which is basic. This basic solution is sodium hydroxide and hydrogen gas. The reaction of sodium and water is exothermic.