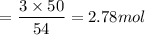Answer: The correct answer is option B i.e., 2.78 mol
Step-by-step explanation:
Aluminium reacts with iodine to form Aluminium iodide
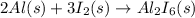
From the equation, it is clear that 3 moles of iodine reacts with 2 moles of Aluminium to form Aluminium iodide
We know
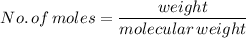
For 2 moles of Aluminium,
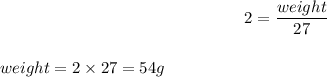
3 moles of Iodine reacts with 54 g of Aluminium
? moles of iodine react with 50 g of Aluminium