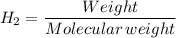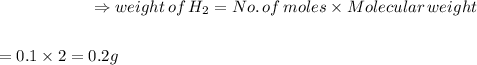Answer:
0.2g of hydrogen is needed to produce 1.80g of water
Step-by-step explanation:
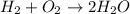
No. of moles of water
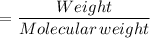
From given values,
Weight of water
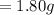
Molecular weight of water
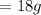
 No. of moles of water
No. of moles of water
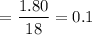
From equation, it is clear that 2 moles of water can be formed by 2 moles of
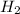
Hence, 0.1 mole of water can be formed from 0.1 mole of
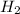
Now,
No. of moles of