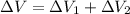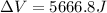Answer:
Step-by-step explanation:
We need to divide the entire process into two different sections, as well
For process 1 we define the following,
It is an Isochoric heating process,
Where
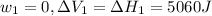
On the other hand for process 2
It is an Isobaric compression process,
Where
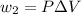
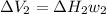
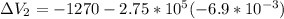
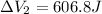
Thus,