Answer:
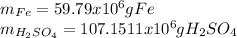
Step-by-step explanation:
Hello,
Based on the given data, the corresponding moles of hydrogen to fill the balloon without loss is:
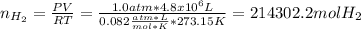
Now, considering the 20% loss of hydrogen, the amount that ensured the complete filling was:
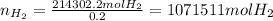
Based on that result, we proceed to computed the amounts of both iron and 98% by mass sulfuric acid that were needed to ensure the complete filling of the balloon as follows:
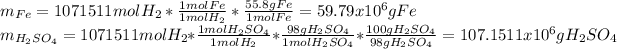
Best regards.