Answer:
Option(a)
The density of 4.5 mL of a liquid that has a mass of 1.3 grams is 0.289g/ml
Step-by-step explanation:
We know that density is given by mass upon volume i.e,
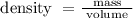
we are given volume of the liquid = 4.5mL
and mass of the liquid = 1.3 grams
So, by substituting the given values in the formula we obtain the density of liquid as
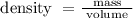
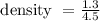
= 0.28888..
= approx 0.289 g/mL which is the density of the liquid