Answer:
1027.62 g
Step-by-step explanation:
For
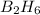 :-
:-
Mass of
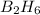 = 296.1 g
= 296.1 g
Molar mass of
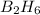 = 27.66 g/mol
= 27.66 g/mol
The formula for the calculation of moles is shown below:

Thus,
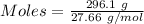
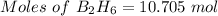
From the balanced reaction:-
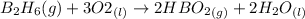
1 mole of
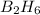 react with 3 moles of oxygen
react with 3 moles of oxygen
Thus,
10.705 mole of
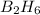 react with 3*10.705 moles of oxygen
react with 3*10.705 moles of oxygen
Moles of oxygen = 32.115 moles
Molar mass of oxygen gas = 31.998 g/mol
Mass = Moles * Molar mass = 32.115 * 31.998 g = 1027.62 g