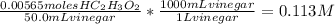Answer:
The concentration of acetic acid present in the vinegar is 0.113M
Step-by-step explanation:
1. As the titration occurs between an acid and a base is called neutralization. The balanced chemical reaction between the acetic acid and the NaOH is:
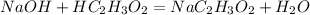
2. Calculate the moles of NaOH used, taking in account the concentration of NaOH 1.00M:
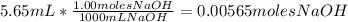
3. Calculate the number of moles of acetic acid neutralized using stoichiometry:
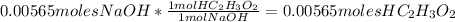
4. Calculate the concentration of acetic acid present in the vinegar: