Answer:
0.881 kilograms
Step-by-step explanation:
Mass of
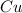 = 305 g
= 305 g
Molar mass of
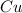 = 63.546 g/mol
= 63.546 g/mol
The formula for the calculation of moles is shown below:

Thus,
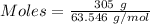
Moles of
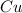 = 4.8 moles
= 4.8 moles
Since in the formula of
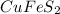 ,
,
1 mole of copper is present in 1 mole of
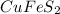
So,
4.8 mole of copper is present in 4.8 mole of
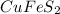
Moles of
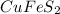 = 4.8 moles
= 4.8 moles
Molar mass of
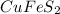 = 183.53 g/mol
= 183.53 g/mol
The formula for the calculation of moles is shown below:

Thus,
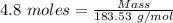
Moles of
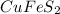 = 881 g
= 881 g
Also, 1 g = 0.001 kg
So,
0.881 kilograms of chalcopyrite must be mined to obtain 305 g of pure Cu.