Answer:
Covalent
Step-by-step explanation:
A molecule of C₂H₅OH has C-C, C-H, C-O, and O-H bonds.
A bond between A and B will be ionic if the difference between their electronegativities (ΔEN) is greater than 1.6.
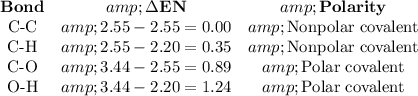
No bond has a large enough ΔEN to be ionic.
C₂H₅OH is a covalent molecule.