Answer:
Original volume=3L
Step-by-step explanation:
Assuming the gas in the ballon is ideal and the temperature does not vary with height we have the gas law as
PV=nRT
Here RHS is constant in both the conditions which means
PV=constant
Given P1=1 atm V1=V P2=0.75atm V2=4L
P1V1=P2V2
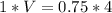
V=3L
The initial volume of the gas is 3L