Answer:
![[NO]_(eq)=0.329M](https://img.qammunity.org/2020/formulas/chemistry/high-school/5lofk3wkmbmx6e0xnf7n2aqxh4yj0io9zk.png)
Step-by-step explanation:
Hello,
The balanced chemical reaction must be stated as follows:
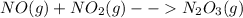
Thus, the equilibrium constant, thanks to the law of mass action turns out being:
![Kc=([N_2O_3]_(eq))/([NO]_(eq)[NO_2]_(eq))](https://img.qammunity.org/2020/formulas/chemistry/high-school/wsidu68i1674ghlknjgk9p08m7jyo2fh71.png)
Based on the equilibrium condition, one leave the law of mass action in terms of the change
 due to the chemical reaction:
due to the chemical reaction:
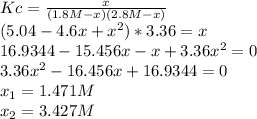
The adequate result is 1.471M since the other one turns out into a negative molarity for NO.
Finally, one conclude that the equilibrium concentration of NO is:
![[NO]_(eq)=1.8M-1.471M=0.329M](https://img.qammunity.org/2020/formulas/chemistry/high-school/zdtux1b6cg6ddhw578br1q3m0ck7ye28wi.png)
Best regards.