Answer: 0.060
Step-by-step explanation:
According to the Dalton's Law, the partial pressure exerted by component 'i' in a gas mixture is equal to the product of the mole fraction of the component and the total pressure.
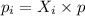
where
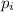 = partial pressure of gas = 0.300 atm
= partial pressure of gas = 0.300 atm
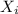 = mole fraction of the component = ?
= mole fraction of the component = ?
p = total pressure = 5.0 atm
Putting in the values we get:
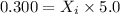
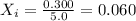
Thus mole fraction of oxygen is necessary in order for the partial pressure of oxygen in the gas mixture the diver breathes to be 0.300 atm is 0.060.