Answer:
a. 4kg/s
b. 99.97 °C
Step-by-step explanation:
Hello,
a. The resulting mass balance turns out into:
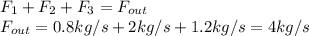
b. Now, the energy balance is:
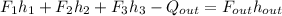
In such a way, the first enthalpy is taken as a liquid-vapor mixture at 1 bar and 0.9 quality, it means:
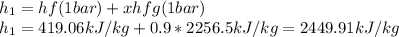
Second enthalpy is taken by identifying that stream as an overheated vapor at 1 bar and 200 °C, thus, the resulting enthalpy is:
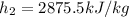
Then, the third enthalpy is taken by considering that at 95°C and 1 bar the water is a saturated liquid, thus:
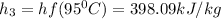 .
.
Now, by solving for
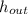 , we've got:
, we've got:
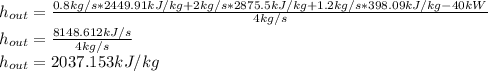
Finally, by searching for that value of enthalpy, one sees that at 1 bar, the exiting stream is a liquid-vapor mixture that is at 99.97 °C and has a 72%- quality.
(NOTE: all the data was extracted from Cengel's book 7th edition).
Best regards.