Answer:
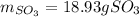

Step-by-step explanation:
Hello,
STP conditions are P=1 atm and T=273.15 K, thus, the reacting moles are:

Now, the balanced chemical reaction turns out into:
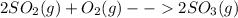
Thus, the exact moles of oxygen that completely react with 0.2366 moles of sulfur dioxide are (limiting reagent identification):
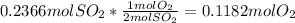
Since 0.2098 moles of oxygen are available, we stipulate the oxygen is in excess and the sulfur dioxide is the limiting reagent. In such a way, the yielded grams of sulfur trioxide turn out into:
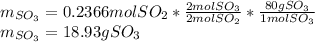
By using the ideal gas equation, one computes the volume as:
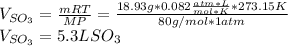
It has sense for volume since the mole ratio is 2/2 between sulfur dioxide and sulfur trioxide.
Best regards.