Answer: a) Volume of
![O_2]](https://img.qammunity.org/2020/formulas/chemistry/high-school/g37yuydk0sj8mccxbqvel1r2swumlcwv4s.png) = 19.2 L
= 19.2 L
b) volume of
 = 20.8 Liters
= 20.8 Liters
Step-by-step explanation:
Combustion is a type of chemical reaction in which fuel is reacted with oxygen to form carbon dioxide and water.
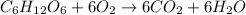
To calculate the moles, we use the equation:
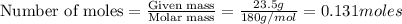
a) Volume of

According to stoichiometry:
1 mole of glucose require = 6 moles of oxygen
Thus 0.131 moles of glucose require =
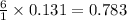 moles of oxygen
moles of oxygen
According to the ideal gas equation:'

P = Pressure of the gas = 1.00 atm
V= Volume of the gas = ?
T= Temperature of the gas = 298 K
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas= 0.783
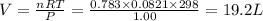
Thus volume of oxygen required is 19.2 Liters
b) Volume of

According to stoichiometry:
1 mole of glucose produce = 6 moles of

Thus 0.131 moles of glucose require =
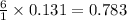 moles of
moles of

According to the ideal gas equation:

P = Pressure of the gas = 0.960 atm
V= Volume of the gas = ?
T= Temperature of the gas =
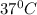 =310 K
=310 K
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas= 0.783
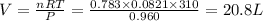
Thus volume of
 produced is 20.8 Liters
produced is 20.8 Liters