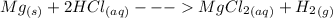Answer:
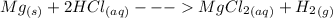
Step-by-step explanation:
The reaction is a simple replacement reaction in which hydrogen gas is liberated when it gets replaced by Magnesium in aqueous solution of hydrochloric acid.
1 mole of Mg reacts with 2 moles of HCl to give 1 mole
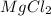 and 1 mole
and 1 mole
 gas. The balanced equation for the reaction Robert observed is as follows:
gas. The balanced equation for the reaction Robert observed is as follows: