The empirical formula : MnO₂.
Further explanation
Given
632mg of manganese(Mn) = 0.632 g
368mg of oxygen(O) = 0.368 g
M Mn = 55
M O = 16
Required
The empirical formula
Solution
You didn't include the pictures, but the steps for finding the empirical formula are generally the same
- Find mol(mass : atomic mass)
Mn : 0.632 : 55 = 0.0115
O : 0.368 : 16 =0.023
- Divide by the smallest mol(Mn=0.0115)
Mn : O =
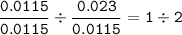
The empirical formula : MnO₂