The no of electrons it have in deficit=1.60
Given:
Charge possessed by a conductor =
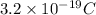
Charge of an electron=
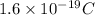
To find:
Amount of electrons in excess or deficit
Step by Step Explanation:
Solution;
Formula used for calculating electrons in excess or deficit is given as
Electrons in excess or deficit= Normal electron charge- Charge of the conductor
Also we know Charge of Conductor
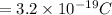 and Electron charge
and Electron charge
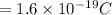
Substitute these values in the above equation we get
Electrons in excess or deficit=
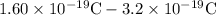
= -1.60
Result:
Hence, it has a deficit of 1.60 electrons.