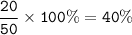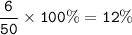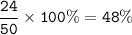The percentage composition of this compound : 40%Ca, 12%C and 48%O
Further explanation
Given
20.0 g of calcium,
6.0 g of carbon,
and 24.0 g of oxygen.
Required
The percentage composition
Solution
Total mass of compound :
=mass calcium + mass carbon + mass oxygen
=20 g + 6 g + 24 g
=50 g
Percentage composition :