Answer:
a) Sm
Step-by-step explanation:
Hello,
This substantiation could be done by knowing how to compute the released energy for 1 mol the oxide. Thus, for Sm, the computation turns out into:
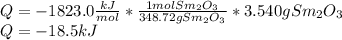
The sign just indicates that the heat is released due to the chemical reaction and it matches with the indicated heat in the statement.
Best regards.