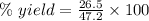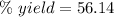Answer:
Step-by-step explanation:
The expression for the calculation of the percentage yield for a chemical reaction is shown below as:-
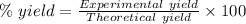
Given , Values from the question:-
Theoretical yield = 47.2 g
Experimental yield = 26.5 g
Applying the values in the above expression as:-