Answer:
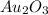
Step-by-step explanation:
Hello,
Based on the initial data, the mass of sample is:
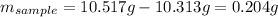
The final mass accounts for the present gold grams into the sample, thus:
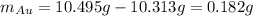
So the oxygen grams are:
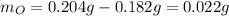
Based on this fact, the moles of both gold and oxygen are:
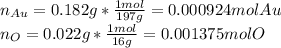
Now, the ratio O:Au allows us to establish the mole relationship between gold and oxygen:
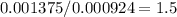
Thus:
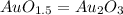
So
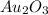 is the empirical formula for the given compound which is auric oxide.
is the empirical formula for the given compound which is auric oxide.
Best regards.