Answer:
C. 10^-7.
Step-by-step explanation:
Hello,
By taking into account that the eventual added ion to the water is removed, it means that after the time goes by, to water will remain pure, so one knows that it implies a pH of 7. Such pH is related with a Ka of 10^-7 based on the mathematical description of pH:
![pH=-log([H^+])](https://img.qammunity.org/2020/formulas/chemistry/high-school/rjvf3gn54iwuwoj9dabpjhdc49gycqpgau.png)
As long as
![[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/jptxicd74sqji6oqiawrnboqrtetqtax3v.png) matches with Ka, it turns out into:
matches with Ka, it turns out into:
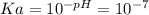
Best regards.