Answer:
Check the procedure out.
Step-by-step explanation:
Hello,
Since neither the mass of magnesium bromide
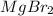 and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into:
and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into:
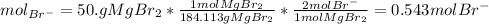
Finally, the required molarity is found:
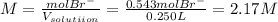
So, if you have a different measured out amount of magnesium bromide and a different volumetric flask, you just modify the 50.0g and 0.250L for the given values you've eventually got.
Best regards.