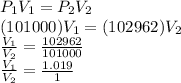Answer:
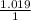
Step-by-step explanation:
To solve this equation we will have to consider that the bubble is filled with an Ideal Gas and as such we can use the Ideal Gas Law
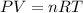
Where
 = Pressure
= Pressure
 = Volume
= Volume
 = Moles
= Moles
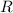 = Ideal Gas Constant
= Ideal Gas Constant
 = Temperature
= Temperature
Now since we know that the value for the temperature and moles is constant we can simply use Boyles Law for the two states
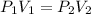
Let us look at the two states
State 1 (at top)
Pressure =

Volume =

State 2 (at bottom)
Pressure =
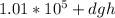
Where
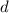 = Density of liquid (1000 kg/m³)
= Density of liquid (1000 kg/m³)
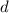 = Acceleration due to gravity (9.8 m/s²)
= Acceleration due to gravity (9.8 m/s²)
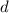 = Height of liquid (0.200 m)
= Height of liquid (0.200 m)
Pressure =
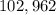
Volume =

Inputting these values into the Boyles Law