Answer:
The minimum speed of a lead bullet is 273.9 m/s
Step-by-step explanation:
Given that,
Initial temperature = 29.5°C
We need to calculate the heat
Using formula of heat
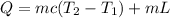
Where,
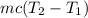 = Energy needed to bring bullet from 29 to 327.3°
= Energy needed to bring bullet from 29 to 327.3°
ml=Energy needed to melt bullet at this temperature
Put the value into the formula
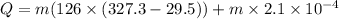
We need to calculate the minimum speed of a lead bullet
Kinetic energy converted to heat energy
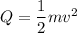
Put the value into the formula
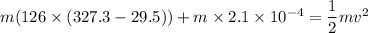
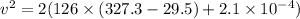
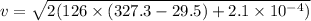
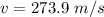
Hence, The minimum speed of a lead bullet is 273.9 m/s