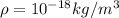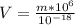To solve the problem we first need to define the nomenclature by which there is an X amount of water in our body. Let's call this a variable m, corresponding to the total mass in us and whose units are Kilograms.
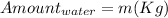
We understand that one millionth of this mass, a small fraction of it, is the equivalent of that of a Water Molecule.
Assuming this to have the same body of water in our body that amount should be approximately
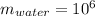
Density of water molecule