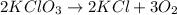Step-by-step explanation:
When a chemical equation has equal number of atoms on both reactant and product side then it is known as a balanced equation.
For example,
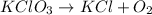
Number of atoms on reactant side are as follows.
K = 1
Cl = 1
O = 3
Number of atoms on product side are as follows.
K = 1
Cl = 1
O = 2
Hence, to balance this equation we multiply
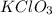 by 2 on the reactant side and multiply KCl by 2 and
by 2 on the reactant side and multiply KCl by 2 and
 by 3 on the product side.
by 3 on the product side.
Therefore, the balanced chemical equation is as follows.