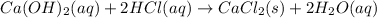Answer : The correct balanced chemical reaction will be:
Explanation :
When calcium hydroxide react with hydrochloric acid then it react to give calcium chloride and water as a product.
This reaction is double-displacement reaction.
Double-displacement reaction : It is defined as the reaction in which the cation of two reactants molecule exchange their places to give two different products.
Thus, the correct balanced chemical reaction will be: