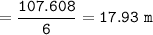The molality of the solution = 17.93 m
Further explanation
Given
6.00 L water with 6.00 L of ethylene glycol(ρ=1.1132 g/cm³= 1.1132 kg/L)
Required
The molality
Solution
molality = mol of solute/ 1 kg solvent
mol of solute = mol of ethylene glycol
- mass of ethylene glycol :
= volume x density
= 6 L x 1.1132 kg/L
= 6.6792 kg
= 6679.2 g
- mol of ethylene glycol (MW=62.07 g/mol)
=mass : MW
=6679.2 : 62.07
=107.608
6 L water = 6 kg water(ρ= 1 kg/L)