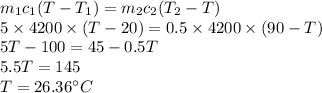Answer:
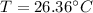
Step-by-step explanation:
Given
mass of the first object
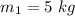
mass of the second object
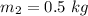
specific heat of the two is
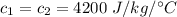
The temperature of the first

The temperature of the second

Heat flows from high temperature to low temperature
Suppose T is the common temperature