Answer:
A) T(t) = 166 degree F
B) Q total = 1196 Btu/min
Step-by-step explanation:
check for characteristics length and Biot number
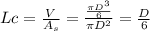
![Lc = ((2)/(12) ft)/(6) = 0.02778 ft</p><p></p><p>[tex]Bi = (hL)/(k) = (42 * 0.02778)/(64.1) = 0.01820 <0.1](https://img.qammunity.org/2020/formulas/engineering/college/q66p0c9hlym9tx0swxw8tg3gyxwdgwibn6.png)
As Biot number is less than 0.1 so it can be determined as lumped parameter system
A) we know that
![b = (hAs)/(\rho Cp V) = \frac{h}{\rho Cp Lc]()
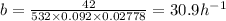
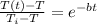
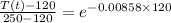
solving for T(t) we get
T(t) = 166 degree F
b) we know that
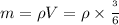
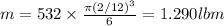
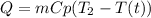
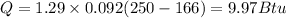
Heat removed from water
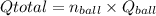
= (120 balls/min)×(9.97 Btu)
Q total = 1196 Btu/min