Answer:
75.51%
Solution and Explanation:
Balanced equation: 6Ag(s) + 2H₃PO₄(aq) → 2Ag₃PO₄(aq) + 3H₂(g)
To determine the percent yield of silver phosphate we will use the following steps;
Step 1: Determine the number of moles of Silver metal used
Mass of silver metal = 50.00 grams
Molar mass of silver is 107.87 g/mol
Therefore;
Moles of silver = 50.0 g ÷ 107.87 g/mol
= 0.464 moles
Step 2: Determine moles of silver phosphate produced
From the equation, 6 moles of Ag reacts with 2 moles of phosphoric acid to yield 2 moles of silver phosphate.
Thus, the mole ratio of silver metal to silver phosphate is 6 : 2
Therefore;
Moles of Ag₃PO₄ = Moles of Ag × 2/6
= 0.464 moles × 2/6
= 0.155 moles
Step 3: Calculate the theoretical mass of silver phosphate produced
Mass = Number of moles × Molar mass
Number of moles of Ag₃PO₄= 0.155 moles
Molar mass of Ag₃PO₄ =418.58 g/mol
Therefore;
Theoretical mass of Ag₃PO₄ = 0.155 moles × 418.58 g/mol
= 64.88 g
Step 4: Calculate the percent yield of Ag₃PO₄
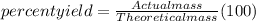
Actual mass of Ag₃PO₄ = 48.99 g
Theoretical mass of Ag₃PO₄ = 64.88 g
Therefore;
% yield of Ag₃PO₄ = (48.99 g ÷ 64.88 g)×100%
= 75.51%
Hence, the percent yield of Ag₃PO₄ is 75.51%