Answer:
The temperature T= 648.07k
Step-by-step explanation:
T1=input temperature of the first heat engine =1400k
T=output temperature of the first heat engine and input temperature of the second heat engine= unknown
T3=output temperature of the second heat engine=300k
but carnot efficiency of heat engine =
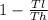
where Th =temperature at which the heat enters the engine
Tl is the temperature of the environment
since both engines have the same thermal capacities
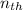 therefore
therefore
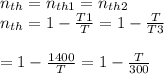
We have now that
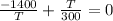
multiplying through by T
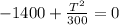
multiplying through by 300
-
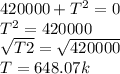
The temperature T= 648.07k