Answer:
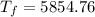 °C
°C
Step-by-step explanation:
Given:
mass of hiker, m= 63 kg
height to be climbed, h= 828 m
energy produced by an energy bar,
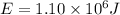
heat capacity of the hiker,
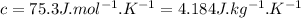
initial body temperature of hiker,
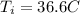
The efficiency of converting the energy content of the bars into the work of climbing is 25%, the remaining 75% of the energy released through metabolism is heat released to her body.
We find the energy required for climbing 828 m height:
W=m.g.h
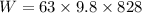
W= 511207.2 J
∵Hike eats 2 energy bars=
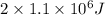
Energy produced

Now, according to her efficiency:
Total energy required for producing the work of W= 511207.2 J which is required to climb the given height will be (say, E):
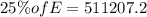
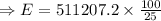
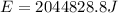
&
Amount of total energy (E) converted into heat(Q) is:
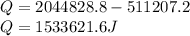
As we know that:
heat,
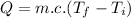 .................(1)
.................(1)
where:
 is the final temperature
is the final temperature
Putting respective values in the eq. (1)
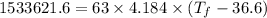
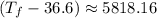
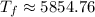 °C
°C