Answer:
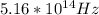
Step-by-step explanation:
1 eV = 1.6 * 10⁻¹⁹ J
The work function is the minimum energy required to cause the photo-emission of electrons. The work function is dependent on the type of metal.
Whereas the threshold frequency is the minimum frequency of light required to produce the work function energy. The work function is given by:
