Answer:
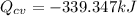
Step-by-step explanation:
First we calculate the mass of the aire inside the rigid tank in the initial and end moments.
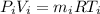 (i could be 1 for initial and 2 for the end)
(i could be 1 for initial and 2 for the end)
State1
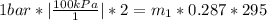
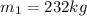
State2
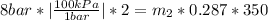
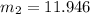
So, the total mass of the aire entered is
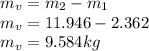
At this point we need to obtain the properties through the tables, so
For Specific Internal energy,
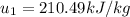
For Specific enthalpy
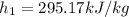
For the second state the Specific internal Energy (6bar, 350K)
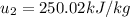
At the end we make a Energy balance, so
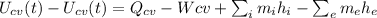
No work done there is here, so clearing the equation for Q
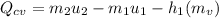
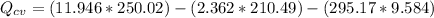
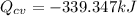
The sign indicates that the tank transferred heat to the surroundings.