Step-by-step explanation:
It is known that relation between kinetic energy and temperature is as follows.
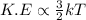
Hence, kinetic energy is directly proportional to temperature.
Thermal energy is defined as the energy present within the molecules of an object due to the motion of particles. Basically, thermal energy is internal energy of an object.
Thus, we can conclude that:
- Temperature and kinetic energy are directly proportional.
- Heat is a measure of thermal energy.
- Temperature is proportional to the total kinetic energy.