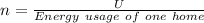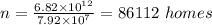Answer:
86112 homes
Solution:
As per the question:
Volume, V =
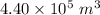
Pressure, P =
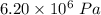
Internal energy, U =
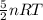
Energy usage of one home = 22.0 kWh
Now,
We know from the ideal gas equation:
PV = nRT
Thus we can write:
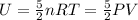
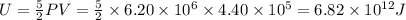
Energy usage of one home =
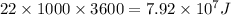
Now,
No. of homes that could serve this internal energy for a day is given by: