Answer:
15 KJ
Step-by-step explanation:
The quantity of heat (Q) required is given as:
Q = mcΔθ + mL
where m is the mass of ice, c is its specific heat capacity, L is its specific latent heat andΔθ is the change in temperature.
Given: m = 20g, temperature of ice = 0
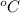 , specific heat capacity of water = 4200 J/kg
, specific heat capacity of water = 4200 J/kg
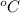 , latent heat of fusion of ice = 3.3 x 10^(5) J/kg, temperature of water = 100
, latent heat of fusion of ice = 3.3 x 10^(5) J/kg, temperature of water = 100
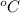 .
.
Q = m (cΔθ + L)
= 0.02(4200 x (100) + 330000)
= 0.02(420000 + 330000)
= 0.02 (750000)
Q = 15000
Q = 15000 Joules
Q = 15KJ
The quantity of heat needed to complete the conversion is 15 KJ.