Moles of Ammonia (NH₃) in solution = 1.14
Further explanation
Given
0.175 L of 6.50 M aqueous ammonia
Required
moles of ammonia
Solution
Ammonia = NH₃
Molarity = moles of solute per liter of solution
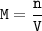
n = M x V
n = 6.50 M x 0.175 L
n = 1.1375 moles
In a multiplication operation: the significant digits in the final product must follow the number of significant digits that are at least in the numbers involved in multiplying
6.50 = 3 sig fig
0.175 = 3 sig fig
So the result must be 3 sig fig
1.1375 rounded to 1.14