Answer:
For 1: The number of oxygen gas molecules used in the reaction are
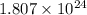
For 2: The number of oxygen gas molecules used in the reaction are
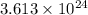
For 3: The moles of aluminium oxide formed are 2 moles
For 4: The mole ratio of Al : O is 4 : 3
Step-by-step explanation:
For the given chemical reaction:
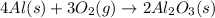
Moles of oxygen gas reacted = 3 moles
According to mole concept:
1 mole of a compound contains
 number of molecules
number of molecules
So, 3 moles of oxygen gas will contain
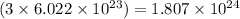 number of molecules.
number of molecules.
Hence, the number of oxygen gas molecules used in the reaction are
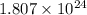
Moles of oxygen gas reacted = 3 moles
1 mole of oxygen gas contains 2 moles of oxygen atoms.
According to mole concept:
1 mole of a compound contains
 number of atoms
number of atoms
So, 3 moles of oxygen gas will contain
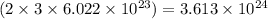 number of oxygen atoms.
number of oxygen atoms.
Hence, the number of oxygen gas molecules used in the reaction are
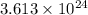
From the given chemical equation, the moles of aluminium oxide formed are 2 moles
Mole ratio is defined as the ratio of moles of substances present in a chemical reaction. It is also the ratio of stoichiometric ratios in a chemical reaction.
Moles of aluminium reacted = 4
Moles of oxygen gas = 3
Mole ratio of Al : O = 4 : 3
Hence, the mole ratio of Al : O is 4 : 3