In a "neutral solution," the [H+] is equal to [OH-] ions.
Option: B
Explanation:
A solution is a chemical mixture of "hydronium" and "hydroxide ions", either in pure water or in any aqueous solution. The properties of solution are dependent on these ions presence and concentration for example if a solution is neutral both the ions have same concentration, in acidic solution hydronium ions concentration is higher while in basic solution hydroxide ions concentration is more. To know the exact presence of such ions the
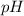 of a solution can be found by using formula,
of a solution can be found by using formula,
![-\log \left[\mathrm{H}_(3) \mathrm{O}^(+)\right]=p H](https://img.qammunity.org/2020/formulas/chemistry/middle-school/9rpq8uue5n5tx6ktv9lg77kjug3efdy2b9.png) .
.