Answer:
The partial pressure of the H(g) that has been formed is 1 atm.
Step-by-step explanation:
Hi, we know that originally we had 0.10 mol of H2(g), but as the reaction progresses, the number of mol in the container changes. So, first, we need to check what is the number of mol in the container when pressure is 3 atm. That is:
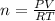
Where:
P=3 atm
V=10L
R= constant
T=3,050K
This should look like this:
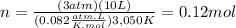
We also know that H2(g) decomposes as follows:
(Equation 1.)
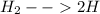
we also know that inside the container there are only mol of H2 and H, therefore:
(Equation 2.)
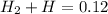
Substitute H2 for 2H and we get
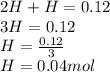
Now, the partial preasure for H would be equals to
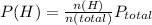
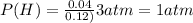
So, the partial preasure of H(g) is 1 atm.
Best of luck.