Answer : The heat released per gram of the compound reacted with oxygen is 71.915 kJ/g
Explanation :
Enthalpy change : It is defined as the difference in enthalpies of all the product and the reactants each multiplied with their respective number of moles. It is represented as
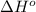
The equation used to calculate enthalpy change is of a reaction is:
![\Delta H^o_(rxn)=\sum [n* \Delta H^o_f(product)]-\sum [n* \Delta H^o_f(reactant)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/2s3ruq22efwpgh8t0j30g9r9s637g4eoxq.png)
The equilibrium reaction follows:
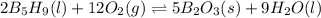
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(n_((B_2O_3))* \Delta H^o_f_((B_2O_3)))+(n_((H_2O))* \Delta H^o_f_((H_2O)))]-[(n_((B_5H_9))* \Delta H^o_f_((B_5H_9)))+(n_((O_2))* \Delta H^o_f_((O_2)))]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ohe7bj1i5za8miczjmxbo66pcnerasr3vr.png)
We are given:
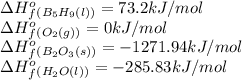
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(5* -1271.94)+(9* -285.83)]-[(2* 73.2)+(12* 0)]=-9078.57kJ/mol](https://img.qammunity.org/2020/formulas/chemistry/high-school/ugjgo2x0qw5f3tw4zysqqajizeo05kg7r8.png)
Now we have to calculate the heat released per gram of the compound reacted with oxygen.
From the reaction we conclude that,
As, 2 moles of compound released heat = -9078.57 kJ
So, 1 moles of compound released heat =
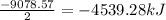
For per gram of compound:
Molar mass of
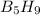 = 63.12 g/mole
= 63.12 g/mole
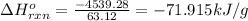
Therefore, the heat released per gram of the compound reacted with oxygen is 71.915 kJ/g