Answer:
The number of D₂O molecules in 300.0 mL of water are 1,003x10²¹
Step-by-step explanation:
First, you need to know in 300 mL of water how many moles of hydrogen and deuterium are. Then, you must use Avogadro's number to obtain the number of molecules.
In 300 mL of water you have:
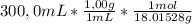 =
=
16,65 moles of H₂O and D₂O
As the 0,01 percent of these moles are D₂O:
16,65 mol × 0,01% = 1,665x10⁻³ mol of D₂O
That in number of molecules are:
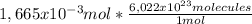 =
=
1,003x10²¹ molecules of D₂O
I hope it helps!