Answer:
0.1388 g
Step-by-step explanation:
The mass of
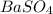 obtained on precipitation = 236 mg
obtained on precipitation = 236 mg
1 mg = 0.001 g
Thus, Mass of
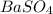 = 0.236 g
= 0.236 g
Molar mass of
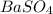 = 233.43 g/mol
= 233.43 g/mol
The formula for the calculation of moles is shown below:

Thus,
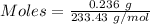
Moles of
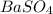 = 0.001011 moles
= 0.001011 moles
According to the reaction,
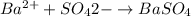
Thus, moles of barium = 0.001011 moles
Molar mass of barium = 137.327 g/mol
Thus, Mass = Moles * Molar mass = 0.001011*137.327 g = 0.1388 g