Answer:
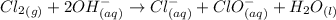
Step-by-step explanation:
Disproportionation is a reaction in which a substance is both oxidized and reduced in the reaction.
Oxidizing agent is the reactant in the chemical reaction which oxidizes others and reduces itself.
Reducing agent is the reactant in the chemical reaction which reduces others and oxidizes itself.
In disproportionation reaction, only one specie acts as both oxidizing as well as reducing agent.
From the given options:-
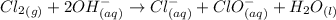
Cl exists in 0 oxidation state in
 and forms
and forms
 in which it has -1 oxidation state and also forms
in which it has -1 oxidation state and also forms
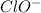 in which it has +1 oxidation state.
in which it has +1 oxidation state.
Thus, Cl is reduced in the chemical reaction as well as it is oxidized too in the chemical reaction.