Answer:
812 kPa
Step-by-step explanation:
- According to Boyle's law pressure and volume of a fixed mass are inversely proportional at constant absolute temperature.
- Mathematically,
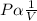
At varying pressure and volume;
P1V1=P2V2
In this case;
Initial volume, V1 = 2.0 L
Initial pressure, P1 = 101.5 kPa
Final volume, V1 = 0.25 L
We are required to determine the new pressure;
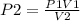
Replacing the known variables with the values;
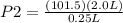
= 812 kPa
Thus, the pressure of air inside the balloon after squeezing is 812 kPa